Coherent Antistokes Raman Scattering (CARS) micro-spectroscopy: Technology development and life-science applications
Optical microscopy is an indispensable tool that is driving progress in cell biology. It is still the only practical means of obtaining spatial and temporal resolution within living cells and tissues.
Most prominently, fluorescence microscopy based on dye-labeling or protein fusions with fluorescent tags is a highly sensitive and specific method of visualising biomolecules within sub-cellular structures. It is however severely limited by labelling artefacts, photo-bleaching and cytotoxicity of the labels. Coherent Antistokes Raman Scattering (CARS) has emerged in the last decade as a new multiphoton microscopy technique suited for imaging unlabelled living cells in real time with high three-dimensional spatial resolution and chemical specificity. This technique has proven to be particularly successful in imaging unstained lipids from artificial membrane model systems, to living cells and tissues to whole organisms.
Activities
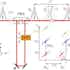
We are actively working on CARS since 2006, and have developed two multiphoton microscopes fully home built (including control software) featuring innovative CARS excitation/detection schemes, significantly improved compared to other systems in the literature in terms of simplicity of realisation and sensitivity. Specifically, dual frequency/differential-CARS (D-CARS) [3] and single-laser CARS [4] were invented and demonstrated utilising femtosecond laser pulses linearly chirped by glass dispersion [1,2].
Beyond technology developments, we are actively working toward the application of CARS to real world biomedical questions in collaboration with the Schools of Biosciences and Medicine. For this purpose we have built a second-generation CARS microscope located in the School of Biosciences. The microscope features enhanced contrast via D-CARS and uses a single 5fs laser system to perform multimodal correlative CARS, two-photon fluorescence and second harmonic generation with a cost-effective and compact design [8].
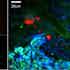
Our biomedical projects currently focus on 1) understanding lipid metabolism in living cells, with particular emphasis on the formation and chemical composition of cytosolic lipid droplets in adipocytes, 2) understanding the lipid distribution and phase segregation in phospholipid membranes, 3) investigating stem cell differentiation and the potential of CARS to determine label-free markers of differentiation, 4) understanding the functional role of lipid droplets in the development of eggs and embryos. We are also developing advanced data analysis tools for phase retrieval of the CARS complex susceptibility, and in turn Raman-like spectra from CARS intensity spectra.
Findings
We have also led the development of a D-CARS module (DCM) as an add-on unit which enables D-CARS microscopy on commercially available two-photon microscopes [9]. The DCM has been successfully tested on a Zeiss LSM510 and routes toward its commercial exploitation are being sought, following the protection of the D-CARS invention with a patent application (PCT/GB2010050473).
Publications
- Di Napoli, C. et al. 2014. Chemically-specific dual/differential CARS micro-spectroscopy of saturated and unsaturated lipid droplets. Journal of Biophotonics 7 (1-2), pp.68-76. (10.1002/jbio.201200197)
- Li, B. , Borri, P. and Langbein, W. W. 2013. Dual/differential coherent anti-Stokes Raman scattering module for multiphoton microscopes with a femtosecond Ti:sapphire oscillator. Journal of Biomedical Optics 18 (6) 66004. (10.1117/1.JBO.18.6.066004)
- Pope, I. et al. 2013. Simultaneous hyperspectral differential-CARS, TPF and SHG microscopy with a single 5 fs Ti:Sa laser. Optics Express 21 (6), pp.7096-7106. (10.1364/OE.21.007096)
- Rocha-Mendoza, I. , Borri, P. and Langbein, W. W. 2013. Quadruplex CARS micro-spectroscopy. Journal of Raman Spectroscopy 44 (2), pp.255-261. (10.1002/jrs.4181)
- Pope, I. et al. 2012. Live cell imaging with chemical specificity using dual frequency CARS microscopy. In: Conn, P. M. ed. Imaging and Spectroscopic Analysis of Living Cells — Optical and Spectroscopic Techniques. Methods in Enzymology Vol. 504.Amsterdam: Elsevier. , pp.273-291. (10.1016/B978-0-12-391857-4.00014-8)
- Langbein, W. W. , Rocha-Mendoza, I. and Borri, P. 2009. Single source coherent anti-Stokes Raman microspectroscopy using spectral focusing. Applied Physics Letters 95 (8) 81109. (10.1063/1.3216073)
- Langbein, W. W. , Rocha-Mendoza, I. and Borri, P. 2009. Coherent anti-Stokes Raman micro-spectroscopy using spectral focusing: theory and experiment. Journal of Raman Spectroscopy 40 (7), pp.800-808. (10.1002/jrs.2264)
- Rocha-Mendoza, I. et al., 2009. Differential coherent anti-Stokes Raman scattering microscopy with linearly chirped femtosecond laser pulses. Optics Letters 34 (15), pp.2258-2260. (10.1364/OL.34.002258)
- Rocha-Mendoza, I. , Langbein, W. W. and Borri, P. 2008. Coherent anti-Stokes Raman microspectroscopy using spectral focusing with glass dispersion. Applied Physics Letters 93 (20) 201103. (10.1063/1.3028346)
Team
-

Head of Condensed Matter and Photonics Group