Improved performance of dry powder inhaler capsules used to treat chronic lung disease
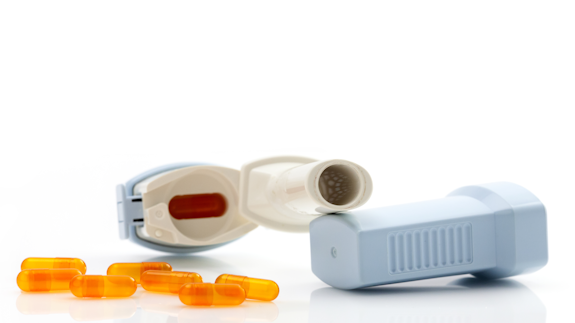
Our research has supported the manufacturing and quality control process of Qualicaps® capsules specifically designed for use in dry powder inhalers.
Working with Qualicaps®, a global market leader in the field of inhalation capsules, Professor James Birchall and Dr Sion Coulman from Cardiff’s School of Pharmacy and Pharmaceutical Sciences developed a test to successfully evaluate the puncture performance of capsules used within dry powder inhalers. Our research has informed Qualicaps®’ manufacturing processes, has helped to dramatically increase sales of their premium products, and enabled the company to enter new markets. In turn, these capsules are being used in dry powder inhalers prescribed to millions of patients with chronic lung disease worldwide.
'We consider the past and present collaboration with Cardiff University as a fundamental factor in our commercial sustainability and success, as well as a key element in our continuous quality improvement efforts.'
How capsules are used in dry powder inhalers to treat chronic lung disease
Every year, millions of dry powder inhalers are prescribed for patients with chronic lung disease, such as asthma.
Many of these inhalers are loaded with a capsule that contains a powdered drug. The capsule is positioned in the inhaler before the patient depresses a button which triggers the movement of steel pins that puncture the wall of the capsule. The powder contained within the capsule then flows through the holes made in the capsule wall and is inhaled into the patient’s lungs to treat the symptoms of lung disease.

Determining how materials and environmental conditions affect the puncture performance of capsules used within dry powder inhalers
Our team of researchers developed the first test of its kind to determine how capsule material and environmental conditions could affect a capsule's performance when it is punctured to release the drug contained within it.
They demonstrated a maintained quality of puncturing performance at low humidity for Qualicaps® premium capsules made from hydroxypropylmethylcellulose (HPMC) compared with the capsules made from gelatin. The team also showed that HPMC capsules are able to maintain quality puncture performance when stored at low temperatures.
These were crucial findings for Qualicaps®. The test provided accurate data to share with clients who had expressed concern over the effects of low humidity on the performance and reliability of their dry powder inhalation formulations to be used inside dry powder inhaler capsules.

Informing product development, supporting company growth and improving capsule performance for patients
The Quality Control Team at Qualicaps® used findings from our capsule performance test to enhance their products. Our findings also influenced Qualicaps®' new investment into the research and development of their inhalation products.
In addition, our research has steered Qualicaps®' marketing and sales strategy to support the company's growth. For example, Qualicaps® continues to use our data to present at major international conferences and cites our findings in its marketing materials to attract new business.

Our research helped Qualicaps® to realise a 91% increase in the sale of their premium HPMC capsules specifically for use in dry powder inhalers. Our findings also informed the company's move towards promoting and selling these premium capsules to clients in new and emerging markets worldwide. Cardiff research provided robust evidence that Qualicaps®' premium inhalation-grade capsules would be punctured effectively in a range of temperatures and different levels of humidity giving Qualicaps® confidence in selling their products in countries where environmental factors were a concern.

‘This research contributes to our improved understanding of how capsules and dry powder inhalers can be used to help treat chronic lung disease. We are proud that our findings have influenced the manufacturing and performance of capsules made by one of the world’s largest suppliers of inhalation products.'
Key facts
- Cardiff researchers tested the puncture performance of capsules made by Qualicaps®, one of the world’s biggest suppliers of capsules for use in inhalation devices.
- Qualicaps® has used our findings to inform its marketing and sales strategies and our data has influenced the company’s investment into the research and development of their inhalation products.
- Millions of chronic lung disease patients who use Qualicaps®’ capsules could benefit from enhanced product performance based on Cardiff’s research.
Meet the team
Publications
- Chong, R. H. et al. 2016. Evaluating the sensitivity, reproducibility and flexibility of a method to test hard shell capsules intended for use in dry powder inhalers. International Journal of Pharmaceutics 500 (1-2), pp.316-325. (10.1016/j.ijpharm.2016.01.034)
- Torrisi, B. M. et al. 2013. The development of a sensitive methodology to characterise hard shell capsule puncture by dry powder inhaler pins. International Journal of Pharmaceutics 456 (2), pp.545-552. (10.1016/j.ijpharm.2013.08.011)
- Birchall, J. C. , Jones, B. and Morrissey, A. 2008. A comparison of the puncturing properties of gelatin and hypromellose capsules for use in dry powder inhalers. Drug Development and Industrial Pharmacy 34 (8), pp.870-876. (10.1080/03639040801928903)




