Exploiting Powder X-ray Diffraction to Complete the Set of Crystal Structures of the 20 “Common” Amino Acids
13 March 2015
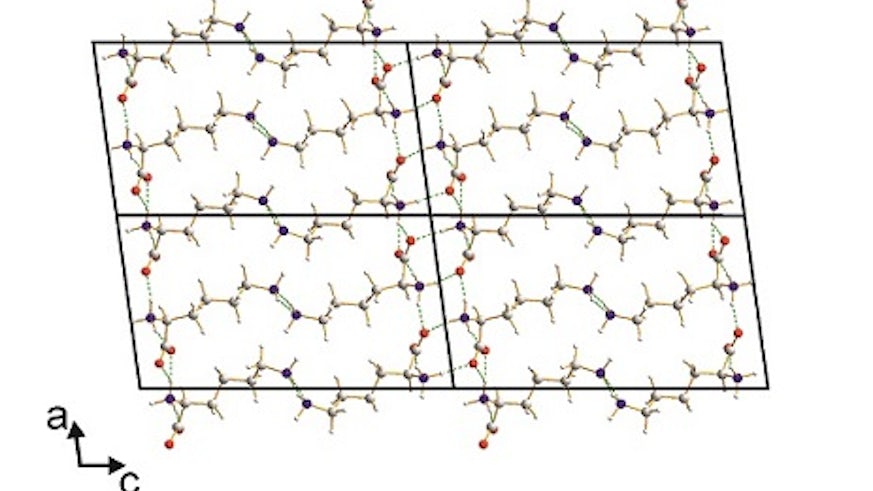
The crystal structure of L-lysine, the final member of the set of the 20 "common" amino acids to have its structure determined, has just been published in Angewandte Chemie International Edition (DOI: 10.1002/anie.201411520) by the Harris group from Cardiff School of Chemistry.
Although it has long been established that all the proteins of life are constructed from a set of 20 "common" amino acids as their basic building units, remarkably the crystal structure of one of these amino acids (L-lysine) had never been reported. The fact that the crystal structure of L-lysine has remained so elusive is because of the challenge of growing single crystals of L-lysine that are of sufficient size and quality for single-crystal X-ray diffraction experiments, which is the standard technique for determining crystal structures. To circumvent this problem, the crystal structure of L-lysine has been determined by exploiting new techniques for the analysis of powder X-ray diffraction data that are being developed at Cardiff Chemistry in the Harris group. These techniques allow complete structure determination to be achieved in cases for which the material of interest can be prepared only as a microcrystalline powder. The publication of the crystal structure of L-lysine completes the set of structures of the 20 "common" amino acids, a quest first started 75 years ago with the first publication of the crystal structure an amino acid (glycine), thus giving the opportunity to understand the solid-state structural properties of all members of this biologically important class of compounds.