A Window to the Past
13 October 2014
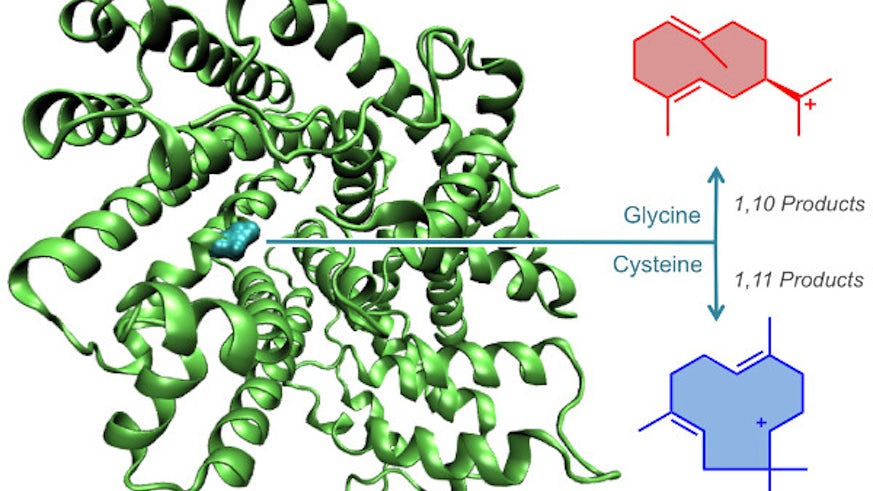
A recent publication in the Journal of the American Chemical Society gives insight into the evolutionary history of plant enzymes.
Plants are a rich source of high value natural products such as terpenes that have many applications as pharmaceuticals, agrochemicals, fragrances and flavours. Terpenes are produced as complex chemical blends reflecting adaptation to selective stresses over evolutionary timescales. With origins in the biosynthesis of hormomes, they have expanded into a wide and structurally diverse collection of specialised natural products with a myriad of important biological functions and applications. Terpene synthases are key enzymes in isoprenoid metabolism; they represent an example of how structural diversity and complexity is generated in nature from a small substrate pool and with only a limited set of biocatalysts.
Work in the research group of Prof Rudolf Allemann has shown that this structural diversity is linked to a single amino acid residue within the active site of terpene synthases. Using a combined synthetic organic chemistry, molecular biology and biophysics approach, the team uncovered an unprecedented molecular switch connecting reaction selectivity and function of sesquiterpene synthases that had previously been thought to have evolved along independent pathways. This functional switch is simply turned on/off by a single mutation. More generally, these new findings suggest that highly specific enzymes such as terpene synthases may have evolved from less specific ancestors through only a small number of amino acid substitutions.