Breakthrough in activating greener chemical reactions
12 October 2023
Cardiff and Swiss researchers develop an electrical “switch” that controls chemical reactions.
A University of Geneva (UNIGE) research group led by Professor Stefan Matile, and Thomas Wirth, professor of organic chemistry at Cardiff University, have discovered how to use an external electric field to control and accelerate a chemical reaction like a ‘‘switch’’.
The work could have considerable impact on the development of new molecules, enabling not only more environmentally-friendly synthesis, but also very simple external control of a chemical reaction.
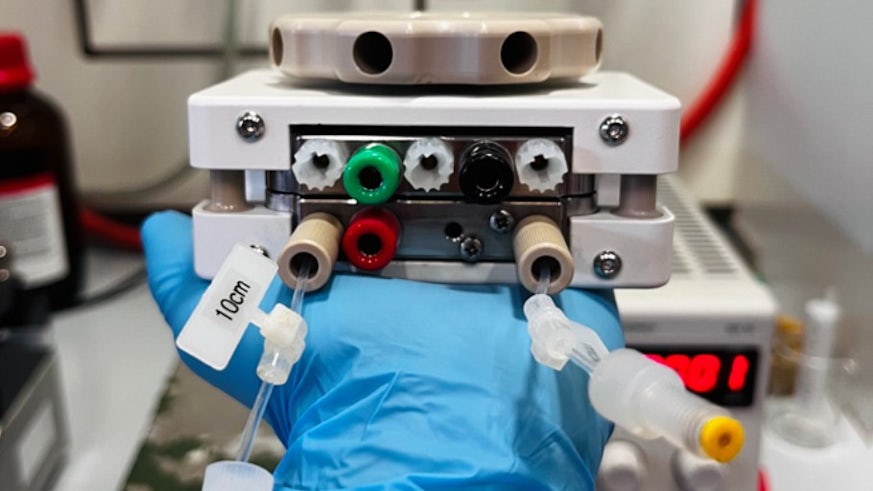
Using a device known as an electrochemical microfluidic reactor, which was designed by Cardiff’s Wirth group, the researchers were able to activate a chemical reaction simply by flipping a switch. The results of the study clearly show the dependence between the state of progress of the chemical reaction and the intensity of the applied electric field.
Professor Wirth said: “The use of an electrochemical flow reactor to control reactions intensively investigated by the group of Professor Matile in Geneva is a real breakthrough in controlling reactions by an electric field, which now allows the production of larger amounts of target molecules”.
Professor Matile said: ‘‘Any molecular transformation results from electrons - negatively charged elementary particles - moving from one place in a molecule to another. Our ‘reactor’ is in some ways like the particle accelerator at CERN in Geneva, but instead of accelerating subatomic particles, it accelerates electrons during molecular transformations.’’
To develop new pharmaceuticals, cleaner fuels and biodegradable plastics needed for a greener future, it’s vital for chemists to create new synthesis methods – giving the ability to change molecules from their natural state into new, more suitable materials.
In chemistry, creating complex organic chemical compounds from simpler reagents is known as organic synthesis. Through successive reactions, chemists assemble small molecules to form new products.
These successive steps are extremely precise and often delicate to control, requiring optimal yields of each reaction step to limit the resources needed. Achieving better control and simpler operation of these reactions poses a major research challenge.
The new electrical device could simplify these strategies, reducing the carbon impact of chemical syntheses, as well as having the advantage of being easy to control. Although previously attempted, this approach had, until now, only resulted in poorly-performing implementations.
Ángeles Gutiérrez López, UNIGE PhD student and first author of the paper said: ‘‘This type of reactor takes the form of a small box in which the reaction mixture can circulate between two electrodes producing the electric field. The electrodes are 5 cm x 5 cm square plates placed as close together as possible. They are separated by a quarter-millimetre-thick sheet. This sheet contains the flow channel for circulating the molecules between the electrodes”.
The electrodes are coated with carbon nanotubes. As they flow through the reactor, the reactants interact weakly with the carbon nanotubes, exposing them to the electric field. This induces electronic polarization in the molecule, activating the chemical transformation.
Fundamental advances are still needed to unlock the device’s full potential. However, this method could be applied to organic chemistry in the not-too-distant future, making the production of drugs, new fuels or new plastics greener and more controllable.
The paper is published in the journal Science Advances.