Researchers marry synthetic biology with nanoscience in fight against resistance to antibiotics
6 October 2021
Antimicrobial Resistance (AMR) is a real threat to society, so new approaches for detecting its cause are needed
AMR occurs when bacteria evolve over time and no longer respond to antibiotic medicine, making illnesses harder to treat and leading to increased risk of mortality.
School of Biosciences researchers Dr Ben Bowen, Ms Rebecca Gwyther and Dr Dafydd Jones, along with collaborators at Queen Mary University of London, have taken a step towards addressing this issue by marrying synthetic biology with nanoscience.
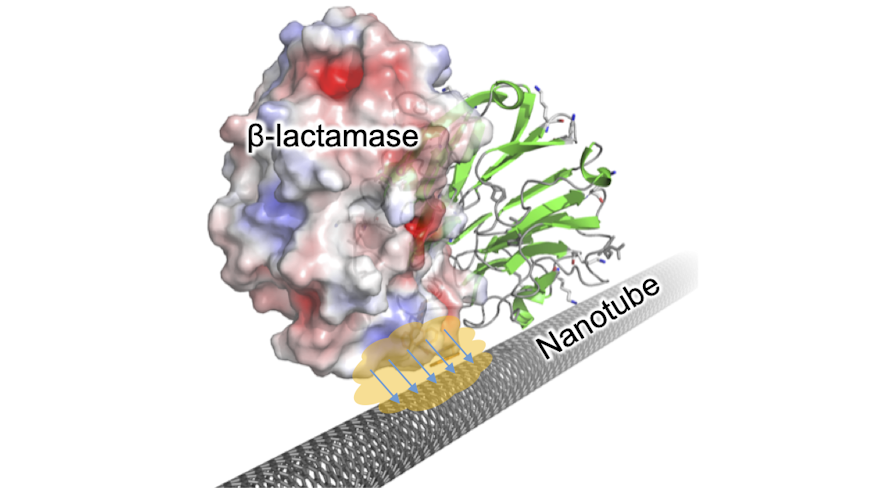
The team are able to detect just a few molecules of an enzyme group, which is the main contributor to bacterial resistance to antibiotics. Known as beta-lactamases, these enzymes break down the penicillin family of antibiotics - the most widely used and most useful of all antibiotics.
They used a protein called BLIP - a universal “antibody” for many different types of beta-lactamase – which they attached to carbon nanotubes to detect its presence. When beta-lactamase molecules were found, they triggered a real-time change in electrical current on the nanometre scale.
Crucially, the electrical response was dependent on the surface of the incoming beta-lactamase, which could potentially allow different versions of the enzyme with different antibiotic degrading ability to be identified, leading to more effective antibiotic treatment.
Dr Jones said: “Engineering biology for uses outside of the biological context has huge implications for future tech. Here we have shown how this can be applied to generate systems on the nanometre scale capable of detecting the causes of AMR within a few minutes and with high sensitivity.”
The findings have been published in the highly prestigious chemistry journal Angewandte Chemie Int Ed.