Improving diagnosis of colorectal cancer
20 February 2019
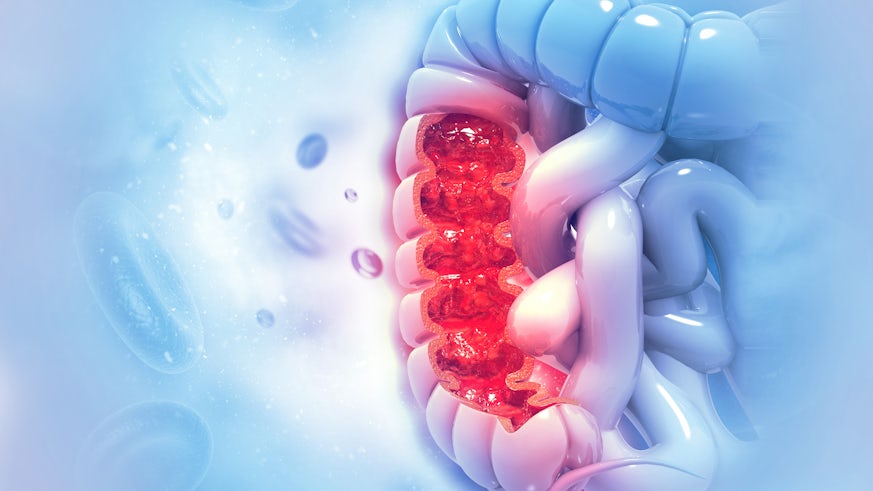
Current faecal blood tests for colorectal cancer have a sixty percent false positive rate. New funding from Cancer Research UK is helping scientists at Cardiff University to find better, safer tests.
Inaccuracy of initial tests for colorectal cancer are putting patients at unnecessary risk, highlighting vital need for the development of precise and non-invasive testing. A grant of over £400,000 is helping researchers at the European Cancer Stem Cell Research Institute to bring this closer to reality.
Dr Lee Parry, Cardiff University’s European Cancer Stem Cell Research Institute, said: “Fifty percent of colorectal cancer cases are preventable.
“Intestinal stem cells are the cells that originate colorectal cancer, and we are investigating how factors like diet, gut bacteria and environment impact on these cells. By understanding the behaviour of these cells, we can improve prevention, diagnosis and treatment of this type of cancer.
“We believe that we can use a biological agent called SL7207 to detect whether colorectal cancer cells are present. We can administer this agent and if this is persistently present in the faecal testing, this is indicative of pre-cancerous colonic polyps.”
Cancer Research UK are providing the European Cancer Stem Cell Research Institute a grant of £498,000, to fund this research.
The Institute at Cardiff University focuses on investigating cancer stem cells - cells in the tumour that initiate the development of cancer and the spread of cancer through the body - aiming to apply their research to transform the way we prevent, diagnose and treat cancers.
The study is in collaboration with Professor Paul Dyson, Swansea University, and Dr Sunil Dolwani from Cardiff University’s School of Medicine.
“If we can prove that we are correct, this will drive the development of a simple to use, safe and cost-effective test for colorectal cancer that can be used in the clinic," added Dr Parry.
“This will also help to reduce burden on hospitals through unnecessary testing, waiting times and risk to healthy patients.
“Being tested for cancer can be a stressful and worrying time, and it is important that these processes are made as efficient and accurate as possible.
“This funding is enabling us to potentially provide effective testing kits for colorectal cancer in the future, which will have a dramatic impact on the testing and diagnosis of this disease."
Share this story
We use our knowledge to develop innovative research that will have an impact on the world.